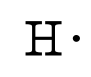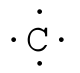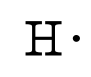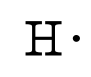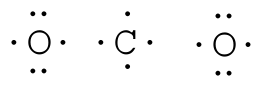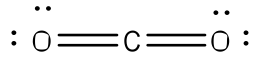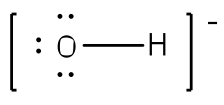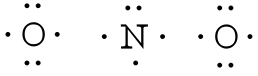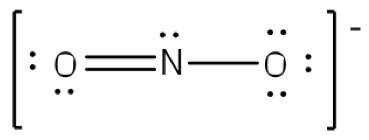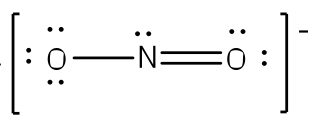Lewis diagrams, also called electron-dot diagrams, are used to represent paired and unpaired valence (outer shell) electrons in an atom. For example, the Lewis diagrams for hydrogen, helium, and carbon are
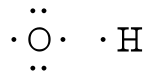
where the symbol represents the element (in this case, hydrogen, helium, and carbon) and the dots represent the electrons in the outer shell (in this case, one, two, and four). These diagrams are based on the electron structures learned in the Atomic Structure and Periodic Table chapters.
The Lewis structure is used to represent the covalent bonding of a molecule or ion. Covalent bonds are a type of chemical bonding formed by the sharing of electrons in the valence shells of the atoms. Covalent bonds are stronger than the electrostatic interactions of ionic bonds, but keep in mind that we are not considering ionic compounds as we go through this chapter. Most bonding is not purely covalent, but is polar covalent (unequal sharing) based on electronegativity differences.
The atoms in a Lewis structure tend to share electrons so that each atom has eight electrons (the octet rule). The octet rule states that an atom in a molecule will be stable when there are eight electrons in its outer shell (with the exception of hydrogen, in which the outer shell is satisfied with two electrons). Lewis structures display the electrons of the outer shells because these are the ones that participate in making chemical bonds.
How to Build a Lewis Structure?
For simple molecules, the most effective way to get the correct Lewis structure is to write the Lewis diagrams for all the atoms involved in the bonding and adding up the total number of valence electrons that are available for bonding. For example, oxygen has 6 electrons in the outer shell, which are the pattern of two lone pairs and two singles. If the electrons are not placed correctly, one could think that oxygen has three lone pairs (which would not leave any unshared electrons to form chemical bonds). After adding the four unshared electrons around element symbol, form electron pairs using the remaining two outer shell electrons.
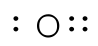 | 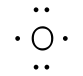 |
| Incorrect Structure | Correct Structure |
are two hydrogen atoms and one oxygen atom. The Lewis structure of each of these atoms would be as follows:
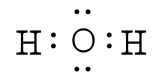
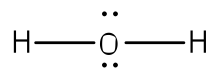
One good example is the water molecule. Water has the chemical formula of H
2O, which means there
We can now see that we have eight valence electrons (six from oxygen and one from each hydrogen). With few exceptions, hydrogen atoms are always placed on the outside of the molecule, and in this case the central atom would be oxygen. Each of the two unpaired electrons of the oxygen atom will form a bond with one of the unpaired electrons of the hydrogen atoms. The bonds formed by the shared electron pairs can be represented by either two closely places dots between two element symbols or more commonly by a straight line between element symbols:
Let us try another one.
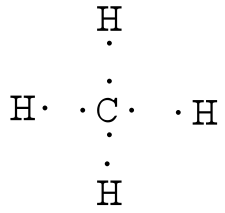
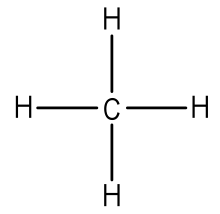
Example: Write the Lewis structure for methane (CH4).
Answer: Hydrogen atoms are always placed on the outside of the molecule, so carbon should be the central atom.
After counting the valence electrons, we have a total of 8 [4 from carbon + 4(1 from each hydrogen] = 8.
Each hydrogen atom will be bonded to the carbon atom, using two electrons. The four bonds represent the eight valence electrons with all octets satisfied, so your structure is complete.
Example: Write the Lewis structure for carbon dioxide (CO2).
Answer: Carbon is the lesser electronegative atom and should be the central atom.
After counting the valence electrons, we have a total of 16 [4 from carbon + 2(6 from each oxygen)] = 16.
Each oxygen atom has two unshared electrons that can be used to form a bond with two unshared electrons of the carbon atom, forming a double bond between the two atoms. The remaining eight electrons will be place on the oxygen atoms, with two lone pairs on each.
Lewis Structures of Polyatomic Ions
Building the Lewis Structure for a polyatomic ion can be done in the same way as with other simple molecules, but we have to consider that we will need to adjust the total number of electrons for the charge on the polyatomic ion. If the ion has a negative charge, the number of electrons that is equal to the charge on the ion should be added to the total number of valence electrons. If the ion has a positive charge, the number of electrons that is equal to the charge should be subtracted from the total number of valence electrons. After writing the structure, the entire structure should then be placed in brackets with the charge on the outside of the brackets at the upper right corner.
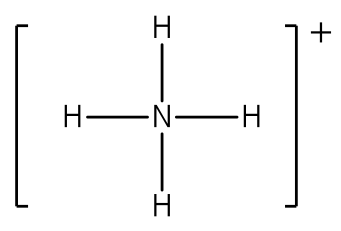
Example: Write the Lewis structure for the ammonium ion (NH4+).
Answer: Hydrogen atoms are always placed on the outside of the molecule, so carbon should be the central atom.
After counting the valence electrons, we have a total of 9 [5 from nitrogen + 4(1 from each oxygen)] = 9. The charge of +1 means an electron should be subtracted, bringing the total electron count to 8.
Each hydrogen atom will be bonded to the nitrogen atom, using two electrons. The four bonds represent the eight valence electrons with all octets satisfied, so your structure is complete. (Do not forget your brackets and to put your charge on the outside of the brackets)
Example: Write the Lewis structure for the hydroxide ion (OH-).
Answer: Since there are only two atoms, we can begin with just a bond between the two atoms.
After counting the valence electrons, we have a total of 7 [6 from Oxygen + 1 from each Hydrogen)] = 7. The charge of -1 indicates an extra electron, bringing the total electron count to 8.
Oxygen will be bonded to the hydrogen, using two electrons. Place the remaining six electrons as three lone pairs on the oxygen atom. All octets are satisfied, so your structure is complete. (Do not forget your brackets and to put your charge on the outside of the brackets)
Lewis Structures for Resonance Structures
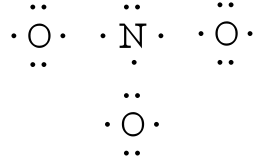
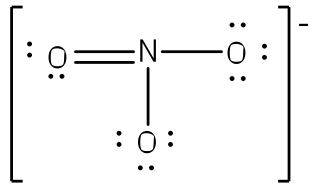
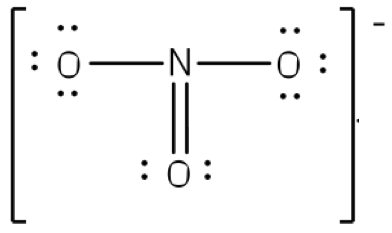
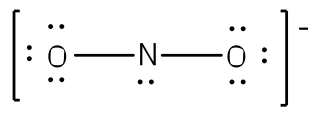
The existence of some molecules often involves two or more structures that are equivalent. Resonance can be shown using Lewis structures to represent the multiple forms that a molecule can exist. The molecule is not switching between these forms, but is rather an average of the multiple forms. This can be seen when multiple atoms of the same type surround the central atom. When all lone pairs are placed on the structure, all the atoms may still not have an octet of electrons. To deal with this problem, the atoms (primarily in a C, N, or O formula) form double or triple bonds by moving lone pairs to form a second or third bond between two atoms. The atom that originally had the lone pair does not lose its octet because it is sharing its lone pair. Double-headed arrows are placed between the multiple structures of the molecule or ion to show resonance. Let us look at how to build a nitrate ion (NO
3-).
Nitrogen is the least electronegative atom and should be the central atom.
After counting the valence electrons, we have a total of 23[5 from nitrogen + 3(6 from each oxygen)] = 23. The charge of -1 indicates an extra electron, bringing the total electron count to 24.
Each oxygen atom will be bonded to the nitrogen atom, using a total of six electrons. We then place the remaining 18 electrons initially as 9 lone pairs on the oxygen atoms (3 pairs around each atom).
Although all 24 electrons are represented in the structure (two electrons for each of the three bonds and 18 for each of the nine lone pairs), the octet for the nitrogen atom is not satisfied. To satisfy the octet rule for the nitrogen atom, a double bond needs to be made between the nitrogen and one of the oxygen atoms. Because of the symmetry of the molecule, it does not matter which oxygen atoms is chosen. Because there are three different oxygen atoms that could form the double bond, there will be three different resonance structures showing each oxygen atom with a double bond to the nitrogen atom. Double-headed arrows will be placed between these three structures. (Do not forget your brackets and to put your charge on the outside of the brackets)
Example: What is the Lewis structure for the nitrite ion (NO2−)?
Answer: Nitrogen is the least electronegative atom and should be the central atom.
After counting the valence electrons, we have a total of 17 [5 from nitrogen + 2(6 from each oxygen)] = 17. The charge of -1 indicates an extra electron, bringing the total electron count to 18.
Each oxygen will be bonded to the nitrogen, using two electrons. Place the remaining 16 electrons initially as nine lone pairs on the oxygen atoms (3 pairs around each atom) and the nitrogen (one pair).
Although all 18 electrons are represented in the structure (2 electrons for each of the two bonds and 14 for each of the seven lone pairs), the octet for the nitrogen atom is not satisfied. To satisfy the octet rule for the nitrogen atom, a double bond needs to be made between the nitrogen atom and one of the oxygen atoms. Because of the symmetry of the molecule, it does not matter which oxygen is chosen. Because there are two different oxygen atoms that could form the double bond, there will be two different resonance structures showing each oxygen atom with a double bond to the nitrogen atom. A double-headed arrow will be placed between these structures. (Do not forget your brackets and to put your charge on the outside of the brackets)
Lewis Structures for Electron-rich Compounds
Elements with atomic number greater than 13 often form compounds or polyatomic ions in which there are “extra” electrons. For these compounds we proceed as above. Once all of the octets are satisfied, the extra electrons are assigned to the central atom either as lone pairs or an increase in the number of bonds. (Never use multiple bonds with these compounds—you already have too many electrons.)
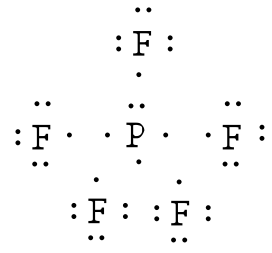
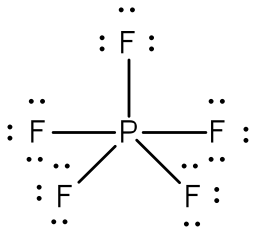
Example: Draw the Lewis structure for phosphorus pentafluoride, PF5.
Answer: The electronegativity of fluorine is greater than that of phosphorus—so the phosphorus atom is placed in the center of the molecule.
The total number of electros is 40 [5(7 from each fluorine) + 5 from the phosphorus] = 40. Using a single bond between the phosphorus atom and each of the fluorine atoms and filling the remaining electrons to satisfy the octet rule for the fluorine atoms accounts for all 40 electrons. Note that there are five bonds around the central atom.
Lewis Structures for Electron-poor Compounds
There is another type of molecule or polyatomic ion in which there is an electron deficiency of one or more electrons needed to satisfy the octets of all the atoms. In these cases, the more electronegative atoms are assigned as many electrons to complete those octets first and then the deficiency is assigned to the central atom.
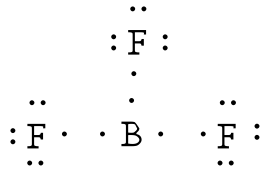
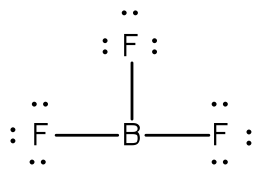
Example: Draw the Lewis structure for boron trifluoride, BF3.
Answer: The electronegativity of fluorine is greater than that of boron—so the boron atom is placed in the center of the molecule.
The total number of electron is 24 [3(7 from each fluorine) + 3 from boron] = 24. Using a single bond between the boron and each of the fluorine atoms and filling the remaining electron as lone pairs around the fluorine atoms to satisfy the octets accounts for all 24 electrons.
The boron atom is two electrons shy of its octet. You may ask about the formation of a double bond (and even resonance). But, fluorine and boron are not in the list that can form double bonds (C, N, O, P, S) and so the compound is electron poor.
Try It Out!
Draw the Lewis structure for the following:
- Hydronium ion (H3O+)
- Hypochlorite ion (ClO-)
- Carbonate ion (CO3-2)
- Ammonia (NH3)
- Hydrogen fluoride (HF)
- Ozone (O3)
- Xenon difluoride (XeF2)